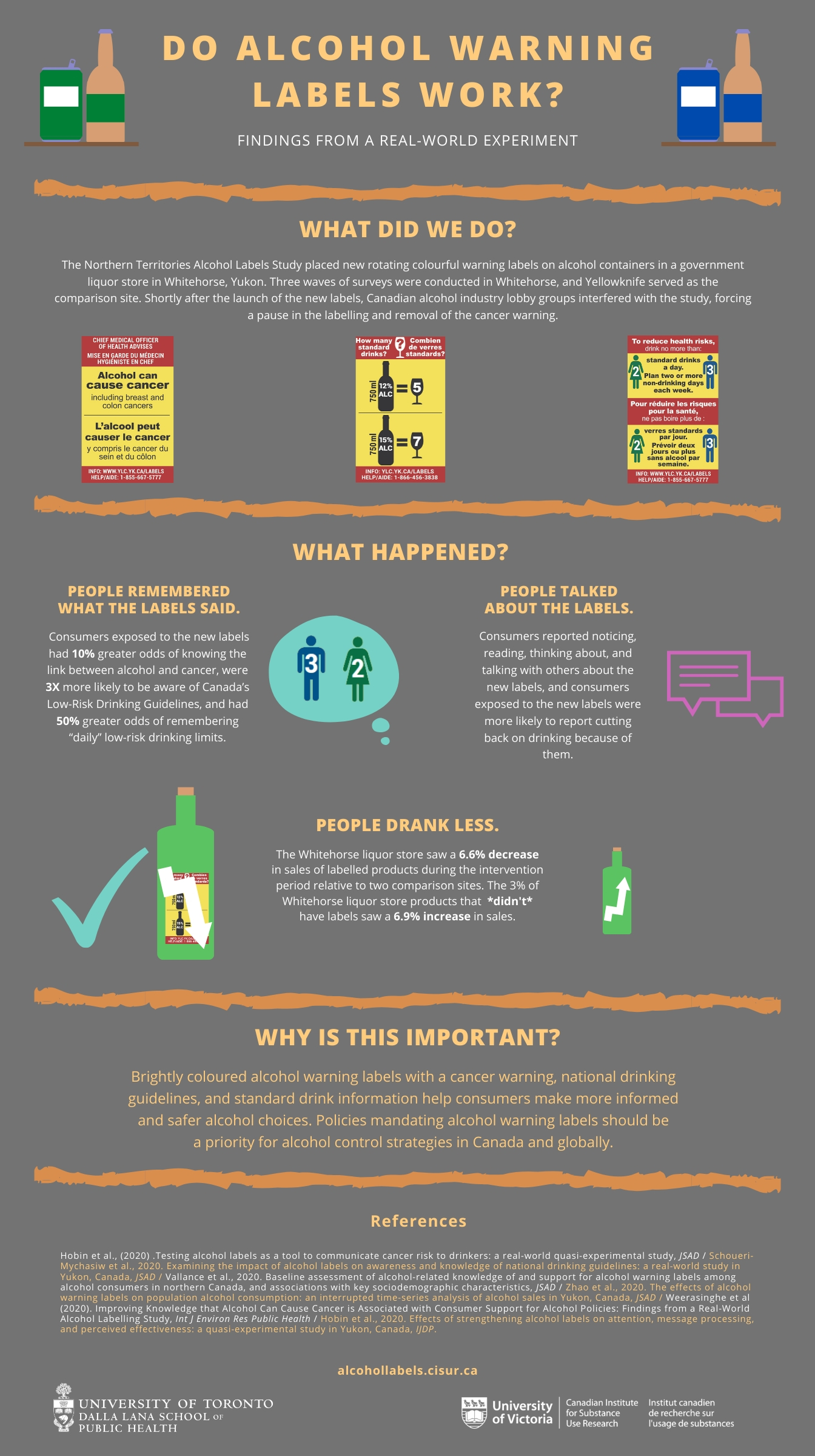
43 warning labels on drugs
A Warning About Warning Labels - U.S. Pharmacist Feb 20, 2009 ... Prescription drug warning labels (PWLs) are applied to prescription vials to provide patients with important instructions for the safe use ... Cigarette Labeling and Health Warning Requirements | FDA Cigarette Packages. Size and location – The required warning must comprise at least the top 50 percent of the front and rear panels of the cigarette package (i.e., the two largest sides or ...
FDA Drug Safety Communication: FDA strengthens warning that ... The U.S. Food and Drug Administration (FDA) is strengthening an existing label warning that non-aspirin nonsteroidal anti-inflammatory drugs (NSAIDs) increase the chance of a heart attack or stroke.

Warning labels on drugs
Defective Drug Warning Labels and Off-Label Use - LawInfo An adequate warning label for a drug will include information such as dosage, active ingredients, and known harmful side effects. However, it's often the case a ... FDA updates warnings for oral and injectable fluoroquinolone [07-26-2016] The U.S. Food and Drug Administration (FDA) approved changes to the labels of fluoroquinolone antibacterial drugs for systemic use (i.e., taken by mouth or by injection). FDA revises labels of SGLT2 inhibitors for diabetes to ... Mar 15, 2022 · A U.S. Food and Drug Administration (FDA) safety review has resulted in adding warnings to the labels of a specific class of type 2 diabetes medicines called sodium-glucose cotransporter-2 (SGLT2 ...
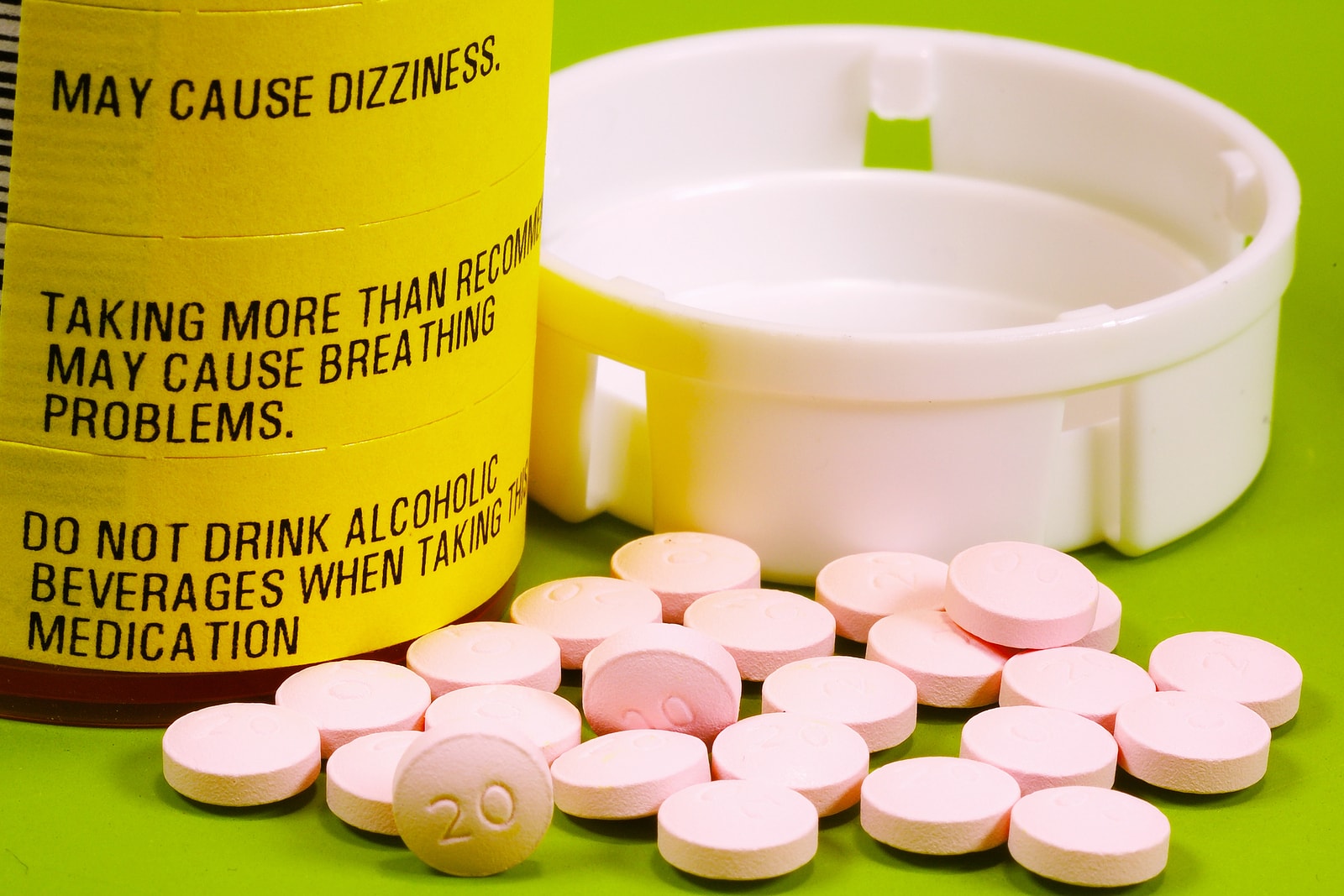
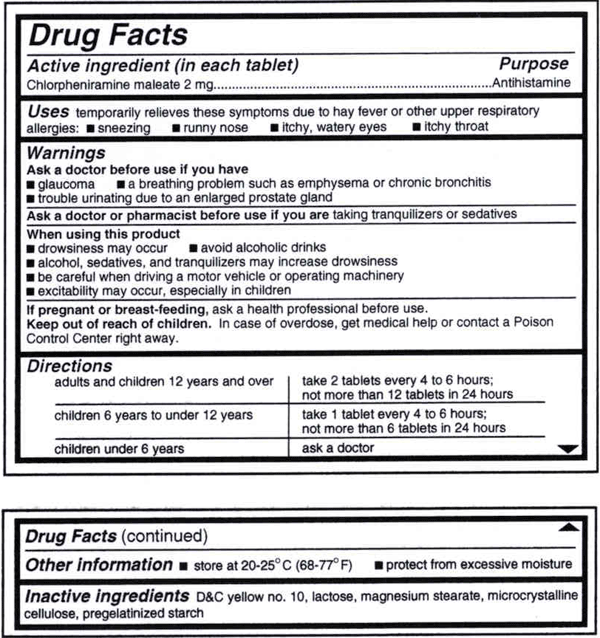
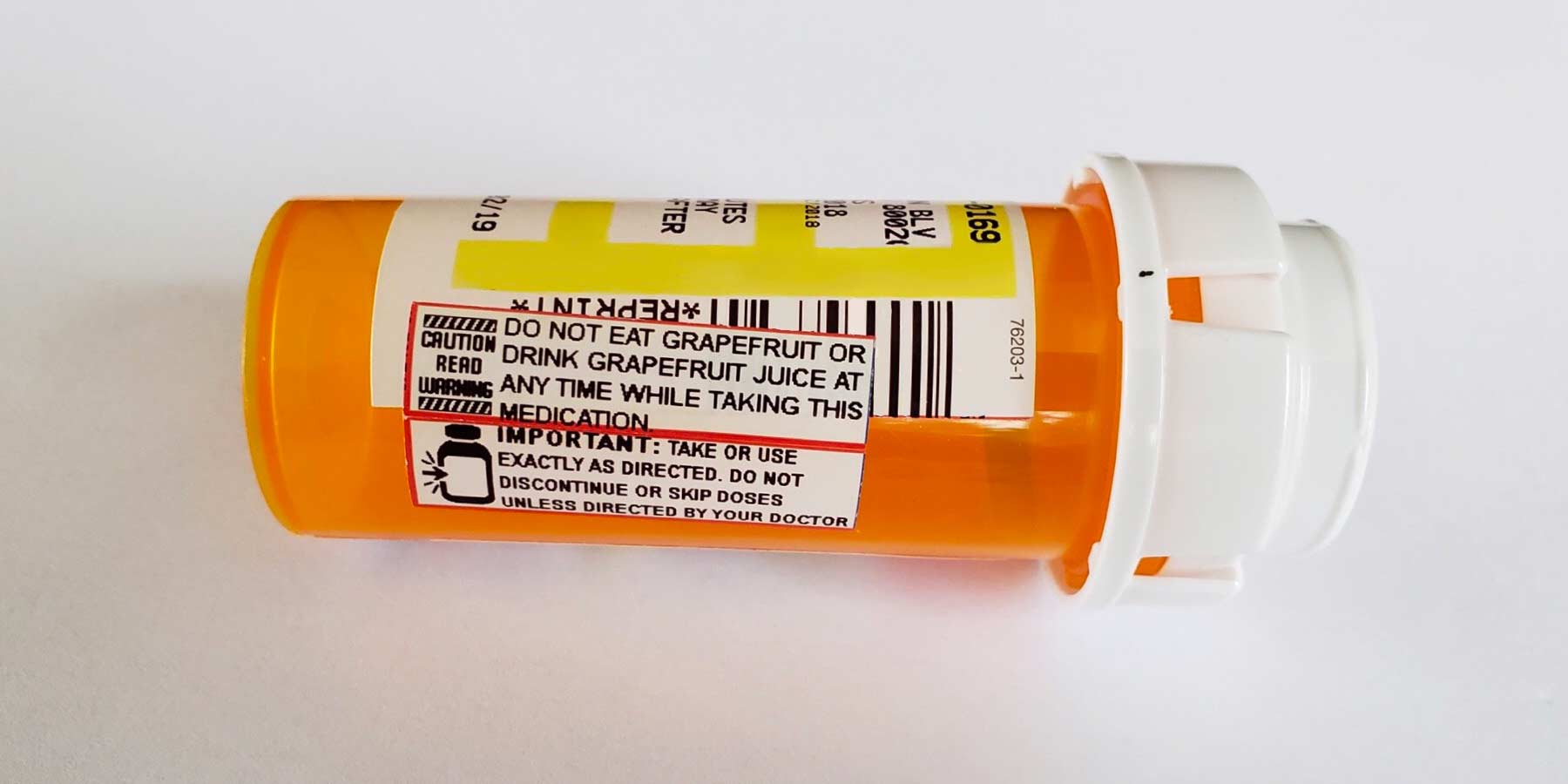
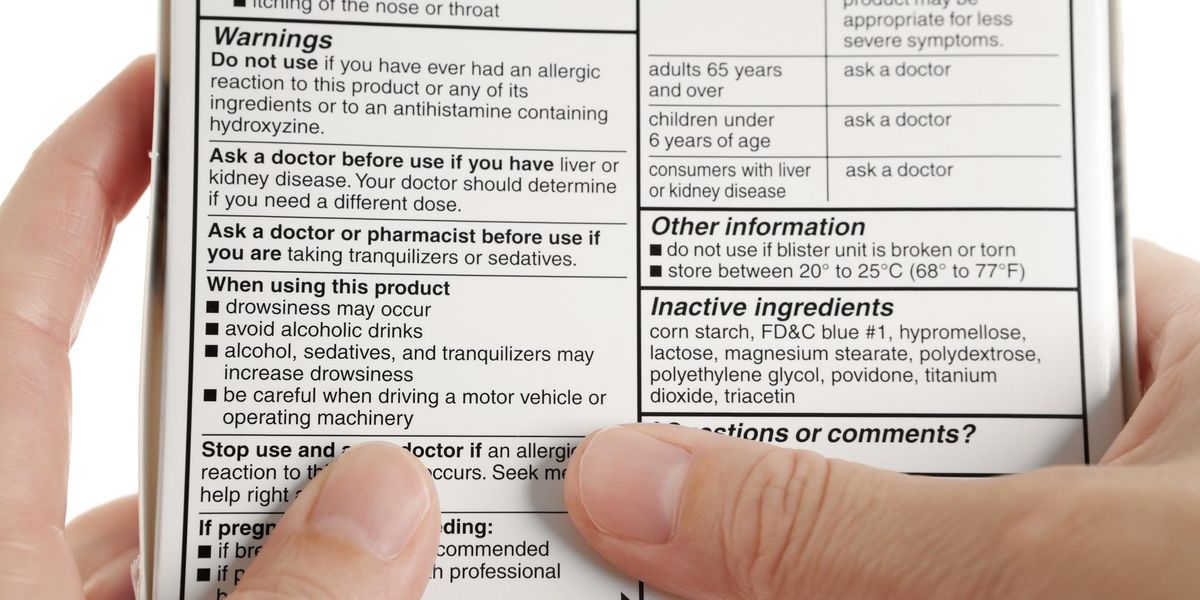
Warning labels on drugs. Prescription Labels and Drug Safety - Consumer Reports No. There are no warnings on the patient drug label itself. Instead, there are two colorful warning stickers placed horizontally on the bottle, separate from ... Perceptions of prescription warning labels within an underserved ... Mar 24, 2014 ... Prescription warning labels are small colored stickers placed adjacent to the drug label on a prescription bottle that provides important ... Black Box Warning - StatPearls - NCBI Bookshelf Jun 23, 2022 · Boxed warnings (formerly known as Black Box Warnings) are the highest safety-related warning that medications can have assigned by the Food and Drug Administration. These warnings are intended to bring the consumer’s attention to the major risks of the drug. Medications can have a boxed warning added, taken away, or updated throughout their tenure on the market. Over 400 different ... What Does it Mean If My Medication Has a 'Black Box Warning'? Jul 24, 2019 ... Black box warnings, also called boxed warnings, are required by the U.S. Food and Drug Administration for certain medications that carry serious ...
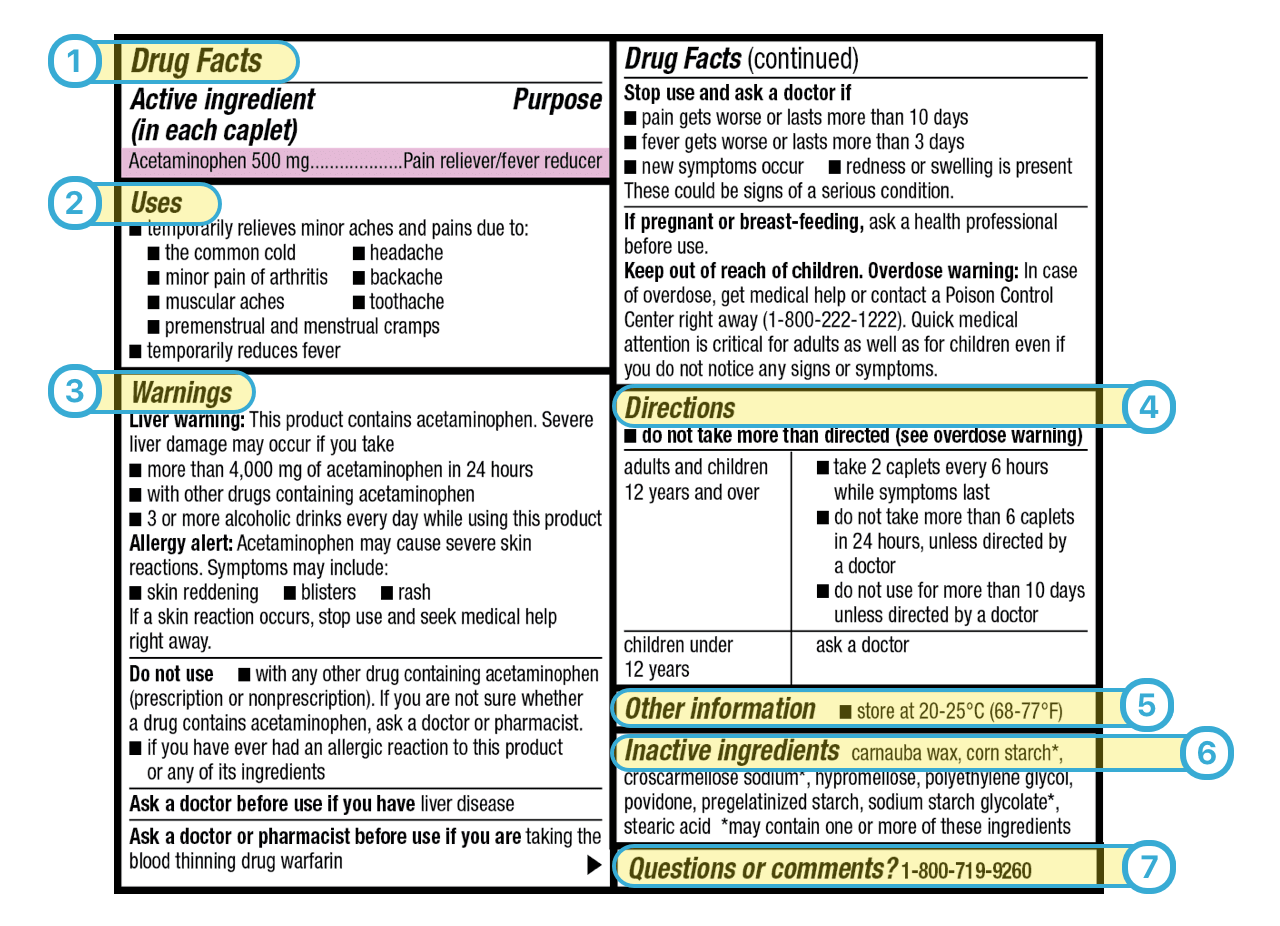
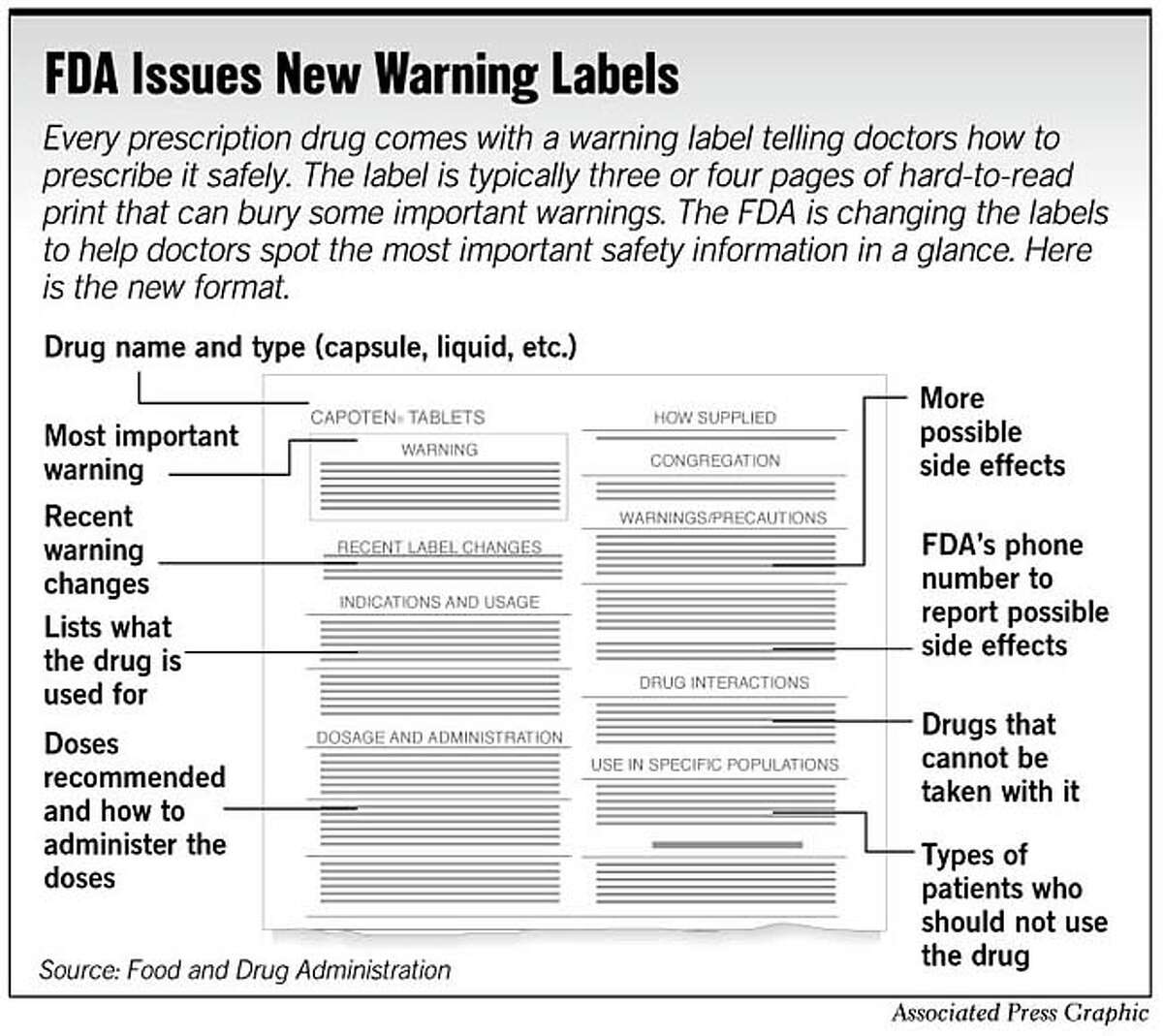
Warning statements for labels and leaflets of certain medicines Dec 29, 2014 ... This guidance sets out the warning statements which should appear on the label and/or in the leaflet of certain medicines. FDA requiring Boxed Warning updated to improve safe use of ... Oct 02, 2020 · To address the serious risks of abuse, addiction, physical dependence, and withdrawal reactions, the U.S. Food and Drug Administration (FDA) is requiring the Boxed Warning be updated for all ... ADDITIONAL WARNING STATEMENTS FOR INCLUSION ... - GOV.UK Where the medicine is a Pharmacy Only (P) medicine this statement must appear on the label: Use this medicine only on your skin. For medicines which are ... How to Read Over-the-Counter and Prescription Drug Labels Warnings. This section is typically the longest section of the Drug Facts label. It tells you about any severe side effects or drug interactions that can occur ...
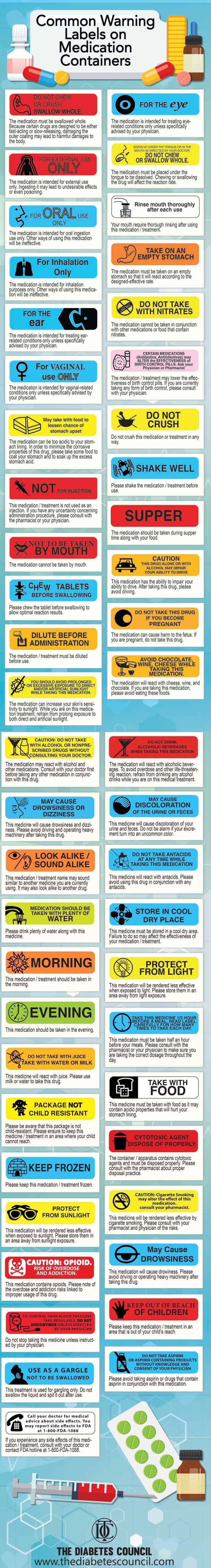
Cautionary and advisory labels | About - BNF - NICE Cautionary and advisory labels · Label 1. Warning: This medicine may make you sleepy · Label 2. Warning: This medicine may make you sleepy. · Label 3. Warning: ... Technology and Science News - ABC News Jul 12, 2022 · Get the latest science news and technology news, read tech reviews and more at ABC News. 50 Common Warning Labels On Medication Containers Jun 2, 2020 ... They are developed to point out critical warnings and information concerning a medication. For some labels, they provide commonly ignored ... FDA revises labels of SGLT2 inhibitors for diabetes to ... Mar 15, 2022 · A U.S. Food and Drug Administration (FDA) safety review has resulted in adding warnings to the labels of a specific class of type 2 diabetes medicines called sodium-glucose cotransporter-2 (SGLT2 ...
FDA updates warnings for oral and injectable fluoroquinolone [07-26-2016] The U.S. Food and Drug Administration (FDA) approved changes to the labels of fluoroquinolone antibacterial drugs for systemic use (i.e., taken by mouth or by injection).
Defective Drug Warning Labels and Off-Label Use - LawInfo An adequate warning label for a drug will include information such as dosage, active ingredients, and known harmful side effects. However, it's often the case a ...


























![PDF] Improving prescription drug warnings to promote patient ...](https://d3i71xaburhd42.cloudfront.net/e087ba4ebaa8ed043bf22bd2ab63292d6a05b9d4/3-Figure2-1.png)


Post a Comment for "43 warning labels on drugs"